Artificial human tissues and organoids or “mini-organs” have potential in biology and biomedicine to help understand cell proliferation, tissue dynamics, and disease. They could potentially be used in regenerative medicine and drug discovery studies. By providing unparalleled complexity, large-scale tissue models are expected to mimic the properties of their human in vivo counterparts, offering a very promising alternative to animal testing.
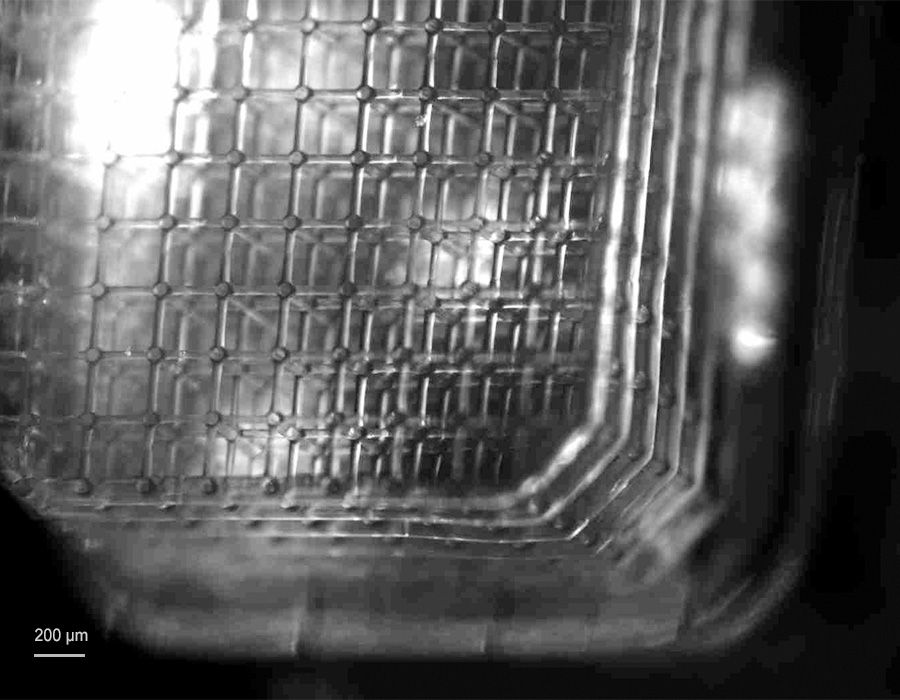
However, microscale vascularization is critical to reproduce the in vivo-like environment in bio-engineered tissues. The presence of a network of microvessels, made up of capillaries, is essential for microcirculation and the exchange of oxygen, nutrients and waste products. Scientists at the KU Leuven have addressed this issue and succeeded in fabricating a 3D soft microfluidic lattice that can perfuse large tissue constructs in the cubic multi-millimeter range. A hydrogel material that is permeable for gases and nutrients was chosen for the fabrication of the soft microfluidic lattices.
3D printing of microvessel networks
Nanoscribe’s Two-Photon Polymerization (2PP) is the key technology that enables the fabrication of a highly interconnected network of synthetic microvessels. The precision capabilities of the 2PP-based 3D printers enabled the fabrication of filigree capillary-like tubes with diameters and thicknesses of a few microns. The printers are also capable to perform this type of fabrication over cubic multi-millimeter volumes. Thus the complex geometry of the vascular network can ensure perfusion over a large three-dimensional space of several tens of cubic millimeters. Importantly, the rapid prototyping capabilities of Nanoscribe’s technology allowed the shape-accurate printing of the CAD geometry, making it the ideal technology for this challenging microfabrication task.
Customized non-swelling hydrogel biomaterial
The clue to the required diffusion characteristics of the vascular network lies in the choice of material. The scientists developed a custom material based on a soft, non-swelling hydrogel that allows the exchange of oxygen, nutrients and waste. The non-swelling properties of the hydrogel are essential to avoid breaking the seal between the soft microfluidic grid and the plastic base on which the grid was 3D printed. The choice of the non-swelling hydrogel is also possible because Nanoscribe’s 3D printers are designed as open material systems that are compatible with the use of customizable, third-party and commercially available materials, extending the flexibility often required in microfabrication.
Accelerated cell differentiation in perfused tissue
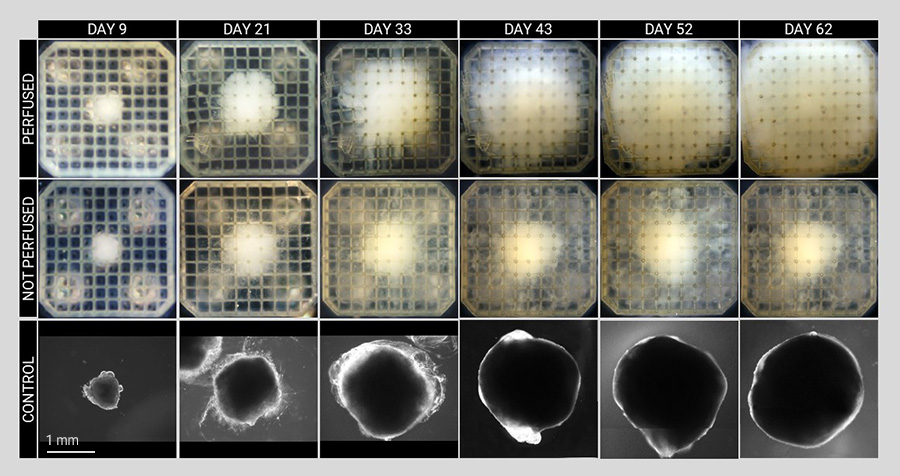
The novel 3D soft microfluidic cell culture platform provides microperfusion for various tissue constructs. Starting from stem cells, the scientists produced cerebral organoids and liver tissue constructs using controlled differentiation, a process through which stem cells acquire their specific roles in the body (e.g., become neurons or hepatocytes). They discovered that microperfusion significantly enhanced tissue growth and accelerated differentiation process compared to non-perfused tissues and conventional cell culturing. Furthermore, the analysis of the perfused neural tissue confirmed the high viability of the tissues over long periods of time.
Would you like to find out more about this inspiring project? Then read the open-access publication here: Large-scale perfused tissues via synthetic 3D soft microfluidics
The complete publication and further scientific publications on hundreds of research projects can be found in a powerful database with an underpinned keyword search in the Premium Resources section. Register for free and gain insights into the potential of Nanoscribe’s 3D Microfabrication technology for innovative applications and fundamental innovations in many areas.